Column: A stem cell clinic tees up a Supreme Court challenge to rules protecting patients’ health and safety

- Share via
For years, the Food and Drug Administration has taken up arms against clinics hawking unproven and ineffective stem cell treatments to desperate patients looking for cures of intractable diseases and conditions such as Alzheimer’s, Parkinson’s, multiple sclerosis and even erectile dysfunction.
As the FDA has repeatedly cautioned, there is no scientifically validated evidence that these treatments work. They’re typically not covered by insurance. For the clinics, however, they’re money-makers, with fees of $9,000 or more per treatment; the clinics often recommend multiple treatments.
But now the FDA’s campaign against these bogus therapies is facing serious headwinds on two fronts.
[The FDA is] likely to be subjected to enormous political pressure during Trump 2.0 to weaken oversight of cell and regenerative products.
— Paul S. Knoepfler, UC Davis
One is the Supreme Court. A California stem cell network that recently lost a lawsuit brought by the FDA has signaled that it intends to appeal to the Supreme Court. It’s far from certain that the court will take up the appeal, at this stage — but a majority of the justices have looked favorably on efforts to rein in administrative agencies such as the FDA.
“I think it’s highly unlikely ... but not impossible” that the court will take up the stem cell case, says Henry T. Greely, an expert in the legal issues involving bioscientific technologies.
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.
The case doesn’t have the customary hallmarks of cases that warrant Supreme Court action, Greely told me, such as disagreements among appellate circuits requiring resolution. But it may suit the ideological bent of four justices — the minimum number required to place a case on the Supreme Court docket.
“Some of these justices really hate administrative agency power,” Greely says.
In a landmark ruling last year, the Supreme Court struck down a 40-year-old precedent—the Chevron doctrine — that required courts to accept federal agencies’ interpretations of the laws they administer as long as their interpretations weren’t openly unreasonable. That sharply narrowed agency authority. The FDA has ranked high on the list of agencies that conservatives see as exercising excessive authority.
It may not take a Supreme Court decision to hamper the FDA’s campaign against bogus stem cell treatments.
The FDA claimed these clinics were plying patients with an illegal drug derived from their stem cells. A federal court agrees.
“Just the possibility that [the Supreme Court] could take this case may have a chilling effect on FDA activity in the stem cell clinic space,” Paul S. Knoepfler, a UC Davis biologist who has assiduously tracked the industry, told me. Even without the case, he says, the FDA is “likely to be subjected to enormous political pressure during Trump 2.0 to weaken oversight of cell and regenerative products.”
That brings us to the second threat, coming from Donald Trump’s nominee as secretary of Health and Human Services, Robert F. Kennedy Jr. Even before his nomination, Kennedy made clear that he was girding to go to war against the FDA, which would come under his jurisdiction at HHS.
In an Oct. 25 tweet, he declared “FDA’s war on public health is about to end.” He specifically accused the agency of “aggressive suppression” of stem cells as well as “psychedelics, peptides, ... raw milk, hyperbaric therapies, chelating compounds, ivermectin, hydroxychloroquine ... and anything else that advances human health and can’t be patented by Pharma.”
Kennedy wasn’t clear what he meant by his reference to stem cells or whether he was referring to the unproven stem cell treatments marketed by the clinics facing FDA regulation.
Many of the other items in his litany have been shown to be ineffective for their marketed purposes — ivermectin and hydroxychloroquine, for example, have been touted as treatments for COVID-19 even though scientific studies have shown them to be useless against the disease. I asked Kennedy to clarify his reference to stem cells but haven’t received a reply.
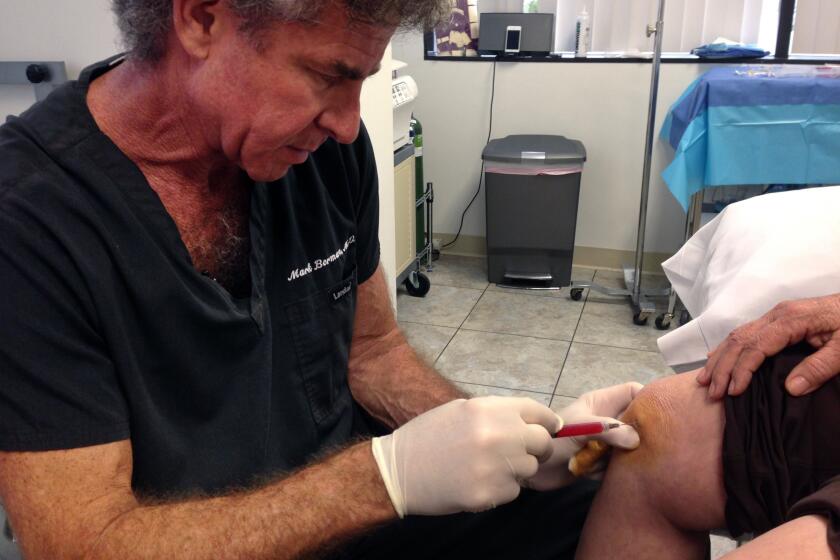
Here’s a brief primer on what these clinics are selling. Typically, their method involves removing fat cells from a customer via liposuction, treating the fat ostensibly to extract stem cells, and injecting those cells into the customer’s body.
For instance, Cell Surgical Network, a defendant in the FDA’s California case, boasts of offering “innovative solutions” for spine disease, knee problems and other orthopedic conditions; lupus, Crohn’s and other autoimmune diseases; ALS, Parkinson’s and multiple sclerosis; cardiac conditions; and glaucoma, among other issues. None of these claims has been supported by scientific research.
The FDA gave stem cell clinics three years to comply with its rules. The number of illegal clinics exploded.
The only stem cell products the FDA has approved for use are stem cells extracted from umbilical cord blood, and then only for rare blood disorders.
Like other clinics, Cell Surgical has asserted that its products are exempt from oversight because, as reimplantations of a customer’s own tissue, they don’t meet the law’s definition of “drugs.”
They also claim the “same surgical procedure” exemption from FDA regulation, which the agency typically applies to procedures in which a patient’s tissue is given only minimal processing before being used, such as in skin grafting or coronary artery bypass surgery. The FDA holds that the stem cell clinics subject the tissues to significant processing and that the procedures are separate surgical events.
Before the FDA acted, both the Florida and California clinic networks had been operating for years. The Florida company had been operating since at least 2014, and Lander and Berman had founded their California Stem Cell Treatment Center in Rancho Mirage in 2010. By 2018, the FDA said in its lawsuit, Lander had claimed that affiliated clinics had administered the technique he and Berman developed to more than 6,000 patients.
Yet the FDA sometimes seems to be fighting a losing battle, or at least a whack-a-mole battle, against clinics offering dubious stem cell treatments. There are just too many — more than 1,000, by Knoepfler’s reckoning — making pitches to desperate customers seeking cures against intractable conditions.
That has left things up to state and local regulators, but the record there is spotty. A notable recent success can be chalked up to Georgia Atty. Gen. Chris Carr, who announced on Jan. 8 that in conjunction with the Federal Trade Commission he had obtained judgments totaling more than $5.1 million from the operators of bogus stem cell clinics. The sum includes refunds of more than $3.3 million for 479 customers, most of whom were “older or disabled adults” who had been “sold expensive, unproven stem cell products.”
In June 2019, federal Judge Ursula Ungaro of Miami ordered U.S. Stem Cell of Florida effectively shut down, siding with the FDA in a lawsuit the agency had filed in May 2018.
The FDA’s case against California-based Cell Surgical Network and its affiliates took a somewhat different course. The agency filed suit in California federal court against the network and its physician-proprietors, Elliott B. Lander and the late Mark Berman, the same day it sued the Florida firm. But it lost at the trial stage in August 2022, when federal Judge Jesus Bernal of Riverside accepted the defendants’ claim that they were entitled to the “same surgical procedure” exemption from FDA oversight.
Clinics with unproven stem cell treatments are already targeting COVID-19 fears.
Bernal’s decision, however, was overturned last September by the San Francisco-based 9th Circuit Court of Appeals, which found in a 3-0 ruling that the FDA’s interpretation of the law “is the only interpretation that makes sense.” The appeals court sent the case back to Bernal with instructions to reconsider the case in light of its finding.
That’s where things stood until Jan. 6, when Cell Surgical Network and its affiliated defendants asked the appellate court to suspend its order to remand the case to Bernal, pending an appeal to the Supreme Court. The FDA opposed the motion, arguing that the Supreme Court is unlikely to take up the case. The appellate court rejected the network’s motion Tuesday, but the network hasn’t indicated that it intends to drop the Supreme Court appeal. I asked its lawyers if their plans have changed but haven’t received a reply.
As I’ve written before, undermining the FDA’s authority has been a right-wing project for years. That’s because the agency’s duty is to stand in the way of businesses desiring to push unsafe and ineffective nostrums at unwary consumers, and also in the way of a perverse idea that personal freedom includes the freedom to be gulled by charlatans.
In 2018, then-President Trump signed a right-to-try law that purportedly gave victims of terminal diseases access to experimental treatments that might save them.
But despite claims that it was designed as a “compassionate measure” for terminal patient, the law was a scam perpetrated by the Koch network and its allies, aimed at undermining the FDA’s authority to make sure our drugs are safe and effective. Sen. Ron Johnson (R-Wis.) ultimately gave the game away, informing then-FDA Commissioner Scott Gottlieb, a critic of the law, that its purpose was to “diminish the FDA’s power over people’s lives, not increase it.”
In 2023, GOP-appointed judges on the right wing-dominated 5th Circuit Court of Appeals ruled that the FDA had exceeded its authority in advising against the use of ivermectin against COVID. “The FDA can inform,” the court said, “but it has identified no authority allowing it to recommend consumers ‘stop’ taking medicine.” (Emphasis in the original.)
There may not be much distance between that finding by the 5th Circuit and a decision that could come from the current Supreme Court majority that the FDA overstepped its bounds by not only informing consumers of the dangers of taking unproven and even dangerous stem cell treatments, but seeking to put clinics that sell them out of business.
“MAGA loves stem cell clinics,” Greely says. “Why? It gives people a chance to make a lot of money, and because it’s a change for people to say ‘no bureaucrat is going to tell me what to do.’”
If the trend continues along these lines, you can expect more providers collecting more dollars by pushing worthless therapies to desperate customers. The threat to Americans’ health will be very real indeed.
More to Read
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.